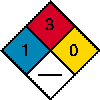
| Lindane Index | Chemistry | Acetone | Benzene |
Solubility in water is 100% at 20°C ( 68.068°F) Vapour irritant to eyes, nose and throat. ROUTES OF EXPOSURE:
Excerpted from MSDS. Human Health Effects: Evidence for Carcinogenicity: CLASSIFICATION: D; not classifiable as to human
carcinogenicity. BASIS FOR CLASSIFICATION: Based on lack of data concerning
carcinogenicity in humans or animals. HUMAN CARCINOGENICITY DATA: None. ANIMAL
CARCINOGENICITY DATA: None. Human Toxicity Excerpts: EFFECTS SIMILAR TO ETHYL ALCOHOL ... BUT ANESTHETIC
POTENCY IS GREATER. 10-20 ML TAKEN BY MOUTH WITHOUT ILL EFFECT. IN ACUTE CASES A
LATENT PERIOD MAY BE FOLLOWED BY RESTLESSNESS AND VOMITING LEADING TO
HEMATEMESIS AND PROGRESSIVE COLLAPSE WITH STUPOR. WORKERS HAVING BEEN EXPOSED TO 1000 PPM, 3 HR/DAY FOR 7-15
YEARS, ALSO COMPLAINED OF CHRONIC INFLAMMATION OF AIRWAYS, STOMACH AND DUODENUM;
SOME OF THEM COMPLAINED OF DIZZINESS & ASTHENIA. SIMILAR COMPLAINTS WERE
REPORTED AFTER EXPOSURE ... TO 700 PPM. PROLONGED OR REPEATED SKIN CONTACT MAY DEFAT THE SKIN
& PRODUCE DERMATITIS. ... Onset of hepatorenal lesions in two men & two
women acutely exposed to acetone /is described/. One person had inhaled acetone
vapors whereas the others had ingested acetone. Clinical manifestation of liver
injury was observed in all four workers & renal lesions were detected in
two. Repeated exposure to 25-920 ppm: chronic conjunctivitis,
pharyngitis, bronchitis, gastritis, and gastroduodenitis. /Route not specified/ SYMPTOMATOLOGY (acute intoxication): 1. Early emotional
lability: exhilariation, boastfulness, talkativeness, remorse, and belligerency.
2. Impaired motor coordination: slowed reaction time, slurred speech, ataxia. 3.
Sensory disturbances: diplopia, vertigo. 4. Flushing of face, rapid pulse,
sweating. 5. Nausea and vomiting. Eventual incontinence of urine and feces. 6.
Drowsiness, stupor and finally coma, with impaired or absent tendon reflexes.
Convulsive episodes may indicate hypoglycemia. /Ethyl alcohol/ SYMPTOMATOLOGY (acute intoxication): 7. Pupils dilated or
normal. 8. Peripheral vascular collapse (shock): hypotension, tachycardia, cold
pale skin, hypothermia. 9. Slow stertorous respirations. 10. Death from
respiratory or circulatory failure or from aspiration pneumonitis. 11. During
convalescence: postalcoholic headache and gastritis; infections (for example,
pneumonia, septicemia); alcoholic psychoses (for example, delirium tremens).
/Ethyl alcohol/ Acute acetone intoxication was reported in a 10-year old
boy who wore a hip cast set with a mixture of 90% acetone, 9% pentane and 1%
methyl salicylate. The following symptoms were described: restlessness,
headache, vomiting (positive benzidine for blood), stupor, blood pressure 80/60,
rapid and irregular respiration rate. A total of 659 males occupationally exposed to acetone and
other solvents were divided into nine unrelated groups working in plastic boat,
chemical, plastic button, paint, and shoe factories. Urine samples were
collected at the beginning of the workshift and at the end of the first half of
the shift. A close relationship (correlation coefficient always above 0.85)
between the average environmental solvent concentration (mg/cu m) measured in
the breathing zone and the urinary concentration of unchanged solvent (ug/l) was
observed. A Biological Equivalent Exposure Limit (56 mg/l) corresponding to the
environmental Threshold Limit Value (58 mg/l) was recommended for acetone. The
biological exposure data for urine collected over 4 hr during random sampling
for at least 1 yr could be used to evaluate long-term exposure and probability
of non-compliance for individual or groups of workers. Direct contact of acetone with the eyes may produce
irritation and corneal injury. High vapor concentrations will produce anesthesia. Acetone can be placed among solvents of comparatively low
acute and chronic toxicities. Acetone does not have sufficient warning
properties to prevent repeated exposures to vapors which may have adverse
effects. There has been no reports that prolonged inhalation of low vapor
concentrations result in any serious chronic effects in humans. Severe toxic effects: 4,000 ppm= 9,650 mg/cu m, 60
minutes; symptoms of illness: 800 ppm= 1,930 mg/cu m, 60 minutes. Toxic concn in human blood: 200.0-300.0 ug/ml (20.0-30.0
mg %); lethal concn in human blood: 550.0 ug/ml (55.0 mg %) Symptoms following acute acetone ingestion include nausea,
vomiting, gastric hemorrhage, sedation, respiratory depression, ataxia, and
paresthesia. Depression resembles alcoholic stupor, but its onset is quicker
than that with ethanol. Coughing and bronchial irritation may be the only clues
to ingestion of quantities that are too small to produce sedation. Hyperglycemia
and ketonemia with acidosis that resembles acute diabetic coma may be present. EXPOSURE FOR 15 MINUTES TO 1660 PPM CAUSES IRRITATION OF
EYES AND NOSE ... Human Toxicity Values: In children 2 to 3 ml/kg is considered to be toxic. Skin, Eye and Respiratory Irritations: EXPOSURE FOR 15 MINUTES TO 1660 PPM CAUSES IRRITATION OF
EYES AND NOSE ... Medical Surveillance: Urinary glucaric acid and the ratio between
6-beta-OH-cortisol and 17-OH-corticosteroids were determined in chemical workers
exposed to styrene greater than or equal to 164 mg/cu m, and acetone greater
than or equal to 571 mg/cu m, and in a control group. Exposed workers had
significantly higher excretion of glucaric acid and a higher ratio. ... Urinary
mercapturic acids were also increased. Simultaneous styrene and acetone exposure
induces mono-oxygenases in humans. ... Probable Routes of Human Exposure: NIOSH (NOES Survey 1981-1983) has statistically estimated
that 1,510,107 workers (466,677 of these are female) are potentially exposed to
Acetone in the US(1). Occupational exposure may be through inhalation and dermal
contact with this compound at workplaces where acetone is produced or used(SRC).
The 8 hour TWA exposure to acetone was in the range of 0-70,000 umols/cu m in a
survey of 659 occupationally exposed male subjects working in shoe, plastics and
chemical plants in Italy (2). Workers in a Japanese acetate fiber producing
plant had detectable levels of acetone in urine samples between 1 and 160
mg/l(3). The average TWA exposure to acetone in 7 spray painting and glue
spraying plants was 0.9, 3.2, 2.3 0.9 and 5.6 ppm for higher-aromatic paint
spraying, lower-aromatic paint spraying, glue spraying, solvent wiping, and
paint mixing respectively(4). The general population may be exposed to acetone through
the use of commercially available products containing this compound such as
paints, adhesives, cosmetics, and rubber cements(SRC). Exposure will also arise
from inhalation of ambient air, ingestion of drinking water, and food that
contains acetone(SRC). The average blood concn of acetone in 600
non-occupationally exposed persons in the US was 3,100 ppb(1). Body Burden: Acetone was detected in the expired breath of 23 of 26
smokers and 42 of 43 nonsmokers in the US(1). Acetone was ubiquitous in the
expired air from a carefully selected urban population of 54 normal healthy
non-smoking people (387 samples) with a geometric mean concn of 101.3 ng/l(2).
Acetone loss in the urine is generally 1 mg/24 hr for a normal adult but is
about 50 mg in children(3,4). Acetone was detected in the expired breath of
children in 2 classrooms in France at an average concn of 800 ng/l(5). Average Daily Intake: AIR INTAKE (assume air concn of 0.05-20 ppb): 24-960 mg;
WATER INTAKE - insufficient data; FOOD INTAKE - insufficient data. (SRC) Note
TOXNET |