By any other name, lindane is the 99% pure gamma isomer of hexachlorocyclohexane. It was introduced as a pediculicide and scabicide in 1952 as Kwell by Reed and Carnrick. The Pharmaceutical Manufacturing Encyclopedia describes the manufacturing process of lindane as one in which chlorine gas is gradually passed into 660 parts of benzene (a known carcinogen) until 890 parts of the gas has been absorbed. The mixture is stirred continuously and the temperature is maintained at 15 degrees C to 20 degrees C. The supply of chlorine is then interrupted and the precipitated solid filtered off and dried. In weight, it is found to be equivalent of 900 parts. The mother liquid is then mixed with 330 parts of benzene and the mixture again treated with 890 parts of chlorine in the manner described. After filtering the reaction mixture resulting from the second chlorination, the filtrate is again mixed with a smaller quantity of benzene and again chlorinated in a similar manner. In this way, a continuous process for the preparation of benzene hexachloride results. This benzene hexachloride isomer mixture is then the raw
material for lindane production. gamma-HCH is isolated by selective
crystallization of crude HCH. Lindane is extracted from HCH by use of
selected solvents, the most common ... is methanol. The gamma-isomer so obtained
is treated with nitric acid to remove odor. Prepared by treating crude benzene
hexachloride with methanol or acetic acid in which the alpha- and beta-isomers
are nearly insoluble, followed by chromatographic adsorption or fractional
crystallization. Benzene + chlorine (photochlorination/isomer
separation). Commercial lindane available in the US contains minimum of
99.9% (by wt) of gamma-isomer. The remaining 0.1% consists of other unspecified
isomers of HCH. Crude benzene hexachloride consists of 10-18% of the
gamma-isomer, 55-70% of the alpha- isomer, 5-14% of the beta-isomer, 6-8% of the
delta-isomer, 3-4% of the epsilon-isomer, and a trace of the eta-isomer. A heptachlorocyclohexane
and an octachlorocyclohexane are present as impurities and contribute to
the unpleasant odor of benzene hexachloride. ITS MODE OF ACTION IS UNKNOWN BUT SPECIFIC
TOXICITY OF GAMMA-ISOMER SUGGESTS THAT ... IT MAY INTERACT WITH PORES OF
LIPOPROTEIN STRUCTURE OF INSECT NERVE CAUSING DISTORTION & CONSEQUENT
EXCITATION OF NERVE IMPULSE TRANSMISSION. European/International
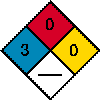
Regulations US DOT Shipping Name: ORGANOCHLORINE PESTICIDE,SOLID,TOXIC (RQ, LINDANE) Hazard Class: 6.1 UN Number: UN2761 Packing Group: III |